From the PED to the CPR
We'll help you meet requirements for selling your industrial products in the EU.
CE Marking and EU Directives
Many industrial and medical equipment sold in the European Economic Area (EEA) must bear the CE marking or the European Conformity Mark.
A CE mark confirms that a product complies with European health, safety, and environmental regulations and can therefore be sold in any EEA country. The European Commission issues Directives for these products and industrial areas, after which individual countries must decide how to implement these Directives in their respective national legislation.
In many cases, manufacturers need an independent body to provide third-party testing, inspection and certification throughout the manufacturing process. These bodies, who are approved by a government and notified to the European Commission, are called Notified Bodies, or NoBos.
We've been a Notified Body for as long as there have been Notified Bodies. LRQA is a NoBo for several EU Directives, and can provide independent certification for your products.
Independence and Impartiality
We maintain our independence and impartiality by proactively managing conflicts of interest across all LRQA businesses including those which may exist between consultancy and third-party certification services. We will not offer consultancy services for any customer product that is in the process of or has completed accredited third-party certification with LR. Furthermore, if we are already providing consultancy services to a customer product, we will not offer any accredited third-party inspection/assessment services for that same product.
Pressure equipment and access
Pressure equipment directive
Simple Pressure Vessels Directive (SPVD) 2014/29/EU
Transportable Pressure Equipment Directive (TPED) 2010/35/EU
Workplace safety
Other products and equipment
Construction Products Regulation (CPR) No. 305/2011
Lifts Directive (LD) 2014/33/EU
ATEX file retention
Online CE Marking Training
Need training on EC Directives and CE Marking? Try our online training that adapts to you and your level of knowledge. Click here for a demo.
Online CE Marking Training
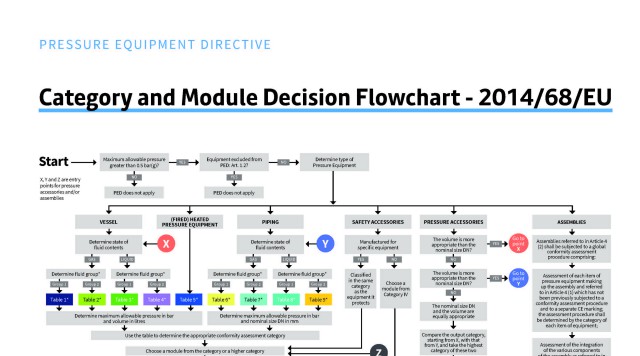
PED Flowchart Poster
Download our free Pressure Equipment Directive flowchart poster to get an overview of how to select your equipment's category and conformity assessment modules.
PED Flowchart Poster